Treatment of Parkinson's disease, oral new drug arrived at the end of phase 3 study
January 31, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Sunovion Pharmaceuticals announced that its key phase 3 clinical trial CTH-300 has reached the primary endpoint and key secondary endpoint of the study. This trial evaluated the effect of apomorphine sublingual membrane (APL-130277) in Parkinson's disease (PD) patients undergoing exercise fluctuations (OFF events).

In the United States, there are more than one million patients with Parkinson's disease (PD), and there are as many as 4 to 6 million patients worldwide. PD is a chronic progressive neurodegenerative disease characterized by tremors, stiffness and impaired movement at rest, and non-motor symptoms including cognitive and mood disorders. It is the second most common neurodegenerative disease after Alzheimer's disease, and the incidence of PD is increasing as the population ages. The OFF event of Parkinson's disease is the re-emergence of PD symptoms in the case of drug control, including both motor and non-motor symptoms. The OFF event may occur at any time of the day, usually after waking up in the morning and periodically during the day. The onset of an OFF event is characterized by tremors, stiffness, or slow movements that can disrupt the patient's ability to perform daily activities, placing a heavy burden on the patient, family, and caregiver. As many as 40%-60% of PD patients will have an OFF event, and the frequency and severity of the attack will worsen during disease progression. These patients urgently need a new drug to effectively control the onset of the OFF event.
The sublingual membrane of Apomorphine (APL-130277) is a new formulation of the fast-acting, easy-to-use dopamine agonist apomorphine, which is expected to help PD patients control OFF events more effectively. Apomorphine is the only on-demand, intermittent treatment approved for the OFF event in patients with advanced PD, but it is currently approved as a subcutaneous injection. Apomorphine sublingual membranes will provide a simpler solution that can be used in the morning and used up to five times a day to help PD patients transition from OFF to ON quickly, safely and reliably. The drug will be used as an on-demand adjuvant treatment for levodopa treatment to help patients control OFF events.
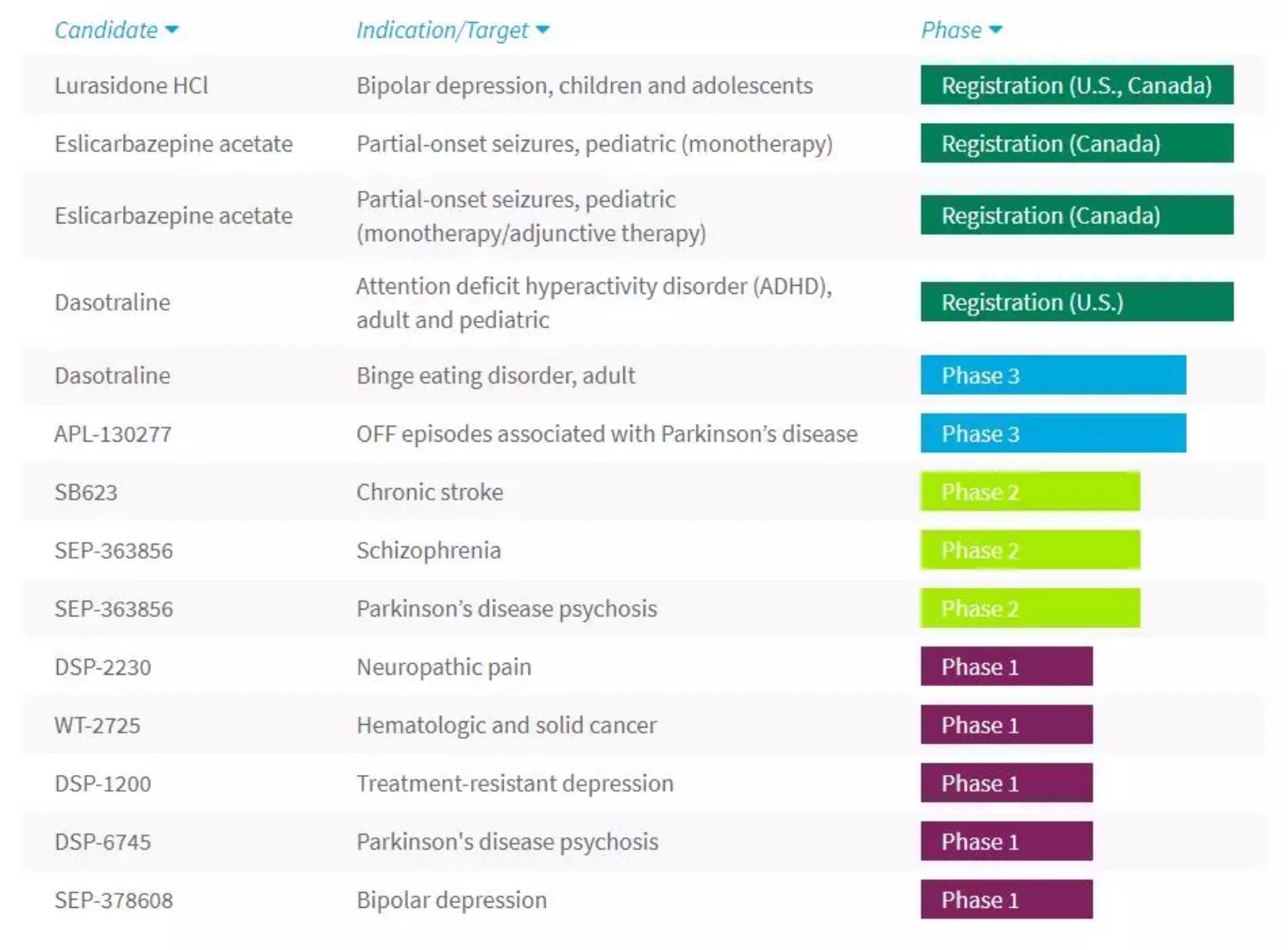
â–² Sunovion Pharmaceuticals R&D pipeline (Source: Sunovion Pharmaceuticals official website)
Apomorphine sublingual membrane (APL-130277) has achieved good results in clinical trials. CTH-300 is a 12-week, randomized, double-blind, placebo-controlled, parallel-group, controlled, phase 3 study. The primary endpoint was the mean change in the MDS-UPDRS (Dynamic Disorders Parkinson's Disease Rating Scale) III exercise examination from pre-dose to 30-minute post-dose during the 12th week of maintenance treatment. The key secondary endpoint was the proportion of patients with complete ON remission assessed by the patient within 30 minutes of the 12th week of the maintenance treatment phase.
Preliminary results showed that the study reached the primary endpoint: the MDS-UPDRS III score in patients receiving apomorphine sublingual therapy was significantly reduced from pre-dose to 30-minute post-dose at week 12 compared with placebo. And the effect continues until the last observation time point is 90 minutes. The difference in MDS-UPDRS III score between the apomorphine sublingual group and the placebo group was 7.6 (p=0.0002). The study also reached a key secondary endpoint: patients treated with apomorphine sublingual membrane had a proportion of patients with complete ON remission within 30 minutes after dosing at week 12 (35% predicted remission rate)) greater than placebo Patient ratio (16% predicted response rate). In addition, the patient was well tolerated by the sublingual membrane of apomorphine.

â–² Dr. Antony Loebel, Executive Vice President and Chief Medical Officer of Sunovion (Source: Sunovion Pharmaceuticals Official Website)
“The OFF event may have serious emotional and practical effects on patients with Parkinson's disease and their families, but there are currently few treatment options for these events,†Sunovion Executive Vice President and Chief Medical Officer, and Sumitomo Dainippon Pharma Group Global Clinical Dr. Antony Loebel, Ph.D., said: "Based on these top-line results, we believe that the apomorphine sublingual membrane may provide a tolerable, reliable, convenient, and rapid onset of treatment for patients with Parkinson's disease who experience an OFF event. select."
"Apomorphine is an effective anti-Parkinson's disease drug, but it is underutilized in patients with Parkinson's disease who experienced an OFF event. Apomorphine is currently only used as an injection. If an alternative method of delivering the drug is approved, For example, apomorphine sublingual membranes will provide an important new option for health care providers and Parkinson's patients,†said Stewart Factor, principal of the CTH-300 study, professor of neurology at Emory University School of Medicine and director of the Movement Disorders Program. "This study shows that the apomorphine sublingual membrane can quickly and safely convert patients with Parkinson's disease from the OFF state to the ON state."
These results will be used to support Sunovion's new drug application (NDA) for the submission of apomorphine sublingual membrane to the US FDA. It is worth mentioning that it has obtained the FDA-issued fast track qualification. We look forward to the launch of this new drug as soon as possible, bringing new treatment options for PD patients.
Reference materials:
[1] Sunovion Announces Positive Topline Results from Pivotal Study of Apomorphine Sublingual Film (APL-130277) in Patients with Parkinson's Disease
[2] SUNOVION ANNOUNCES POSITIVE TOPLINE RESULTS FROM PIVOTAL STUDY OF APOMORPHINE SUBLINGUAL FILM (APL-130277) IN PATIENTS WITH PARKINSON'S DISEASE
[3] Sunovion Official Website
Best Water Distiller,Distilled Water Machine,Podiatry Water Distiller,Portable Water Distiller
ZHEJIANG FOMOS MEDICAL TECHNOLOGY CO.,LTD. , https://www.ifomos.com