Abstract: Objective To establish a method for the determination of airway resistance and airway responsiveness in guinea pigs by using dual-chamber bodyograph, which provides an effective means for the study of asthma. Methods double chamber plethysmography response times were measured in guinea pig airway resistance after methacholine (Mch is) excitation; normal control group were measured airway resistance in guinea pig airway in the first 15 days of the experiment the reaction 2, each time interval 2h, verify the reproducibility of the measurement result; OVA sensitization observed changes in airway resistance and airway reactivity of the guinea pig before and after OVA challenge. Results After the Mch of the PC 100 concentration was atomized, the airway resistance of the guinea pig returned to the baseline level within 1 h . In the normal control group , the basal airway resistance sRaw measured twice on the 1st and 15th days was (3.25±0.67) cmH 2 O·s , (3.33±0.58) cmH 2 O·s , (3.30±0.56) cmH 2 O·s , (3.32±0.75) cmH 2 O·s , airway reactivity (log2PC 100 ) values ​​were 8.48±0.94 , 8.64±1.04 , 8.56±0.67 , 8.64±0.60 , airway resistance and airway reactivity. There was no significant difference between the two comparisons (P>0.05) . The airway resistance and airway responsiveness of the sensitized guinea pigs after OVA challenge were significantly higher than those before the challenge. The sRaw was (7.08±1.82) cmH 2 O·s and (2.87±0.53) cmH 2 O·s (P<0.01) , respectively . The log2PC 100 values ​​were 6.64 ± 1.26 and 8.48 ± 1.17, respectively (P < 0.01) . Conclusion An experimental method for the determination of airway resistance and airway responsiveness in guinea pigs by dual chamber plethysmography was successfully established.
Â
Key words: body scanner; airway resistance; airway reactivity; guinea pig
Guinea pigs are susceptible to sensitization and produce type I and type IV allergies and are among the most commonly used animals to construct asthma models. However, the guinea pig asthma model constructed in the domestic literature often lacks respiratory physiological indexes such as airway responsiveness. Strictly speaking, it can only be regarded as a model of allergic airway inflammation in guinea pigs. Airway hyperresponsiveness (AHR) is one of the basic characteristics of bronchial asthma. To elucidate the pathogenesis of asthma, it is necessary to study the mechanism of AHR . This study was the first in China to determine the airway resistance and airway responsiveness of guinea pigs by dual-chamber mapping. It aims to establish a simple and reproducible method for measuring airway responsiveness and provide an effective means for the study of asthma.
   Â
1 Materials and methods
1.1 Materials
Methocholine ( Mch) , chicken egg albumin (OVA) , aluminum hydroxide purchased from Sigma ; diphenhydramine hydrochloride ( Tianjin Pharmaceutical Group ) ; portable ultrasonic atomizing inhaler ( Yuehua Instrument Factory ) ; double chamber plethysmography (BUXCO Corporation USA); micro ultrasonic nebulizer (Aerogen company USA).
1.2 Animals and grouping
Ordinary white Hartly guinea stage 26, body weight (400 ± 50) g, male, purchased from Guangdong Province Experimental Animal Center, were randomly divided into 3 groups. Normal control group A : 8 guinea pigs, using a series of concentrations of Mch atomized guinea pigs until PC 100 (after atomizing a certain concentration of Mch , the airway resistance increased by 100% or more compared with the basic airway resistance , that is , the atomization Mch was stopped . The concentration is PC 100 ) and then the time taken for the airway resistance to return to baseline levels is observed. Normal control group B : 12 guinea pigs were tested for airway resistance and airway responsiveness on guinea pigs on days 1 and 15 , respectively, at intervals of 2 h . Asthma group C : 6 guinea pigs were tested for airway resistance and airway responsiveness 1 time before and after OVA sensitization , with a time interval of 2 h .
1.3 Experimental methods
1.3.1 Making an Asthma Model The production of an asthma model is based on the literature [1] with minor modifications. Asthma in guinea pigs injected intraperitoneally experiment l containing 5% OVA day and 10 mg / ml aluminum hydroxide in physiological saline 1 ml mixture sensitized guinea pig experimental day 15 placed in a 35 cm × 26 cm × 31 cm of In the plastic box, the portable ultrasonic atomizing inhaler atomized and inhaled 1% OVA physiological saline solution for 2 min to induce asthma attack in guinea pigs, and the atomization speed was 1.3 ml/min . 10 min before nebulization, guinea pigs were injected intraperitoneally with diphenhydramine hydrochloride at 4 mg/kg to inhibit possible hypersensitivity.
1.3.2 Determination of airway resistance and airway reactivity The dual-chamber body scanner consists of a head chamber and a body chamber, each with a flow sensor for measuring airflow changes caused by nasal breathing and airflow changes caused by thoracic motion. The flow sensor sensed by the flow sensor is converted into an electrical signal, amplified by an amplifier, converted into a digital signal, and analyzed by a software (BioSystem XA software with NAM analyzer) to calculate a specific airway resistance ( sRaw) . The guinea pig's neck is covered with three moderately sized rubber mats to hold the guinea pig and maintain a relatively tight seal between the two chambers. The guinea pig was placed in the body spirograph, and the sRaw was dynamically measured after the guinea pig was quiet for 5 min . The micro-ultrasonic atomizer was used to atomize the physiological saline, 25 , 50 , 100 , 200 , 400 , 800 , 1600 mg/L Mch 30 s , the atomization speed was 0.2 ml/min , and the sRaw 2 was monitored after 30 s of atomization. Min . If the sRaw rises less than 100% compared to the base sRaw , the next concentration is atomized. When a certain concentration of Mch is atomized , sRaw rises by 100% or more compared to the base sRaw , and this Mch concentration is determined to be PC 100 . At this point, the atomization Mch is stopped . The greater the PC 100 value of a guinea pig , the lower the airway reactivity of this guinea pig.
1.4 statistical processing
The analysis was performed using SPSS11.0 statistical software, and the PC 100 values measured by each guinea pig were subjected to log2 numerical transformation. The indicators ( measurement data ) were expressed as mean ± standard deviation, and the significance test was analyzed by one-way analysis of variance. The comparison between groups was performed by LSD ( variance of variance ) or Tamhane's T 2 ( when variance was observed ) . The comparison of airway resistance and airway responsiveness before and after OVA- sensitized guinea pig OVA was performed by paired t- test. P < 0.05 was considered statistically significant.
   Â
2 results
In the normal control group, the respiratory wave of the guinea pigs was smooth, and the respiratory wave of the sensitized guinea pig OVA was not significantly different from that of the guinea pigs in the normal control group. The sensitized guinea pig OVA challenged the high wave, the frequency increased, and the airway resistance increased. See Figures 1 and 2 .
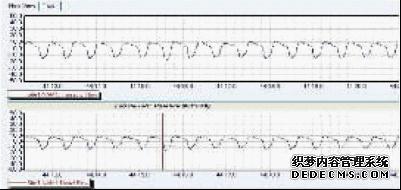
Figure 1 sensitized guinea pig OVA pre-excited respiratory wave
Fig.1 Rspiratory wave of sensitized guinea pigs before OVA challenge
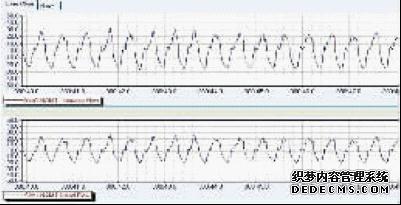
Figure 2: Respiratory waves after sensitized guinea pig OVA challenge
Fig.2 Respiratory wave of sensitized guinea pigs after OVA challenge
2.2 Time required for sRaw to return to basal level after Mch stimulates guinea pigs
After using PC 100 concentration of Mch to atomize guinea pigs, the sRaw of guinea pigs can be increased by 1 time, and the sRaw of some guinea pigs can continue to rise. After 4 minutes , the resistance gradually falls back. Generally , it can fall back to the basic value level within 30 min , No. 6 6 min guinea pig airway resistance i.e. down to basal levels, but 4 airway resistance in guinea pigs 1 h before falling back to basal levels.
2.3 Double chamber mapping instrument for measuring the repeatability of guinea pig sRaw and airway reactivity measurement results
In the normal control group B , the basal airway resistance sRaw was measured on the 1st and 15th days respectively (3.25±0.67) cmH 2 O·s , (3.33±0.58) cmH 2 O·s , (3.30±0.56) cmH 2 O · s, (3.32 ± 0.75 ) cmH 2 O · s, pairwise comparisons of the four time points airway resistance, there is no significant difference (P> 0.05). 4 airway reactivity (log2PC 100) corresponding to the measured values of guinea pigs were 8.48 ± 0.94, 8.64 ± 1.04, 8.56 ± 0.67, 8.64 ± 0.60, which value is pairwise comparisons, there was no statistically significant difference (P> 0.05 ) . This demonstrates the good reproducibility of guinea pig sRaw and airway responsiveness using a dual-chamber stereogram, which allows multiple simultaneous detection of these two indicators in guinea pigs.
2.4 Verifying the asthma model of guinea pigs
Before and after OVA asthma model guinea pigs were excited sRaw (2.87 ± 0.53) cmH 2 O · s and (7.08 ± 1.82) cmH 2 O · s (P <0.01), described before OVA challenge in guinea pigs than OVA challenged airway resistance Airway resistance. The log2PC 100 values before and after OVA challenge were 8.48±1.17 and 6.64±1.26, respectively (P<0.01) , indicating that the airway reactivity after guinea pig OVA challenge was higher than that before OVA challenge. From the perspective of respiratory physiology, the sRaw and airway responsiveness can be effectively verified by a dual-chamber bodyographer .
   Â
3 discussion
Guinea pigs are susceptible to sensitization and can produce type I and type IV allergies, and are particularly suitable for making animal models of allergic asthma. However, the guinea pig asthma model constructed in the domestic literature often lacks the functional indicators of respiratory physiology. Strictly speaking, it can only be regarded as a model of allergic airway inflammation in guinea pigs. Therefore, it is necessary to perform lung function tests on this " allergic airway inflammation " to confirm the successful construction of the asthma model. At the same time, the determination of this respiratory physiological function is conducive to observing the effects of interventions such as drugs, and is conducive to the discussion of the pathogenesis and diagnosis and treatment mechanism of asthma. The laboratory in the domestic first purchase of US BUXCO's dual-chamber plethysmography for airway responsiveness sRaw guinea pigs were measured. Dual chamber plethysmograph system comprises several parts plethysmograph (plethysmograph), an ultrasonic nebulizer (Ultrasonic Nebulizer) atomization and regulation system, the pre-amplifier (MAXII preamplifier unit), a signal processing system (BioSystem XA for Windows software), etc. . The plethysmograph consists of a head chamber ( for measuring changes in nasal airflow ) and a body chamber ( for measuring changes in chest airflow ) , each chamber being connected to a flow sensor to measure changes in respiratory flow in the chamber. The working principle is to calculate the airway resistance sRaw according to the airflow lag time dT of the nasal cavity than the chest cavity . Since the movement of the thorax first generates gas into and out of the body, the airflow of the nose lags behind the airflow of the chest to produce a time difference dT . When sRaw increases, the phase difference between the nasal airflow and the chest airflow increases. According to the formula: sRaw = [(T I + T E) / (2π) (P atm -47) × × 1.36 × 2π × dT / (T I + T E)] (P atm atm behalf equation, T I representatives Inspiratory time, T E stands for exhalation time ) , and sRaw can be calculated by software (BioSystem XA software with NAM analyzer) to understand the airway resistance of animals. The obtained experimental data show that concentrations of 100 in guinea pigs Mch atomizing PC, sRaw guinea pigs can still rise within minutes, within 1 h to return to baseline resistance. To rule out the application of Mch excitation in the assay will affect the next sRaw measurement, the interval between two measurements of sRaw should be more than 1 h , so in order to verify the repeatability of guinea pig sRaw and airway responsiveness measurements by dual-chamber microscopy , experiment 1, 15 days repeated measurements of normal guinea pigs in the control group 1, the measurement time interval chosen to be the same day 2 h. The results showed that there was no significant difference in airway basic resistance and airway responsiveness. It was proved that the double-chamber plethysmography was used to determine the airway basal resistance and airway responsiveness of guinea pigs with good repeatability. The results of this experiment showed that the sRaw and airway reactivity of OVA- sensitized guinea pigs were significantly higher than those before challenge, which proved that OVA sensitization and guinea pig stimulation are simple and effective methods for constructing asthma model. The application of double-chamber plethysmography is asthma research. Provides an effective means.
references:
[1]Zhou Linfu , Yin Kaisheng . Correlation of low-dose arsenic trioxide on airway eosinophil apoptosis and expression of nuclear factor- kappa B in asthmatic guinea pigs [J]. Chinese Journal of Tuberculosis and Respiratory Diseases , 2002, 25(7): 439.
[2] Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals [J]. J Appl Physiol, 1978, 45(4): 644-7.
[3] Petak F, Habre W, Donati YR, et al. Hyperoxia-induced changes in mouse lung mechanics: forced oscillations vs. barometric plethysmography [J]. J Appl Physiol, 2001, 90(6): 2221-30.
[4] Lundblad LK, Irvin CG, Adler A, et al. A reevaluation of the validity of unrestrained plethysmography in mice [J]. J Appl Physiol, 2002, 93(4): 1198-207.
[5] Mitzner W, Tankersley C. Interpreting Penh in mice [J]. J Appl Physiol, 2003, 94(2): 828-31.
[6] Johanson WG Jr, Pierce AK. A noninvasive technique for measure- air of conductance in small animals [J]. J Appl Physiol, 1971, 30(1): 146-50.
[7] Pennock BE, Cox CP, Rogers RM, et al. A noninvasive technique for measurement of changes in specific airway resistance [J]. J Appl Physiol, 1979, 46(2): 399-466.
[8] Song Jianyong , Ren Yuxiang , Lin Hui , et al . Treatment of experimental asthma in mice with PKC inhibitor Rottlerin [J]. Journal of Third Military Medical University , 2003, 25(16): 1411-4.
Stretch Sheet,Non-Woven Stretch Sheet,Disposable Stretch Sheet,Medical Stretch Sheet
Luck Medical Consumables Co.,LIMITED , https://www.luckmedical.com