For CCR4, new lymphoma drugs are eligible for priority review
November 29, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Kyowa Hakko Kirin announced that the US FDA has accepted the mogamulizumab Biologics Licensing Application (BLA) for the treatment of patients with cutaneous T-cell lymphoma (CTCL) who have received at least one systemic treatment and has been granted priority review. .

CTCL is a rare non-Hodgkin's T-cell lymphoma. The two most common types of CTCL are mycosis fungoides (MF) and Sezary syndrome (SS). According to the stage, the disease may involve skin, blood, lymph nodes and internal organs. Late stage CTCL is associated with significant morbidity and mortality. These patients are in desperate need of new treatments to alleviate the disease.
Kyowa Hakko's mogamulizumab, developed using its proprietary POTELLIGENT® platform, is a humanized monoclonal antibody (mAb) targeting CC chemokine receptor 4 (CCR4), which is frequently found in certain hematologic malignancies (including CTCL). Expressed on leukemia cells. Mogamulizumab was first approved for the treatment of other malignant hematologic tumors in Japan in 2012 and was approved for the treatment of CTCL in 2014. The US FDA has awarded mogamulizumab a breakthrough therapy for the treatment of MF and SS patients who have received at least one systemic treatment. The mogamulizumab was once again eligible for priority review, proving its potential in treating CTCL.
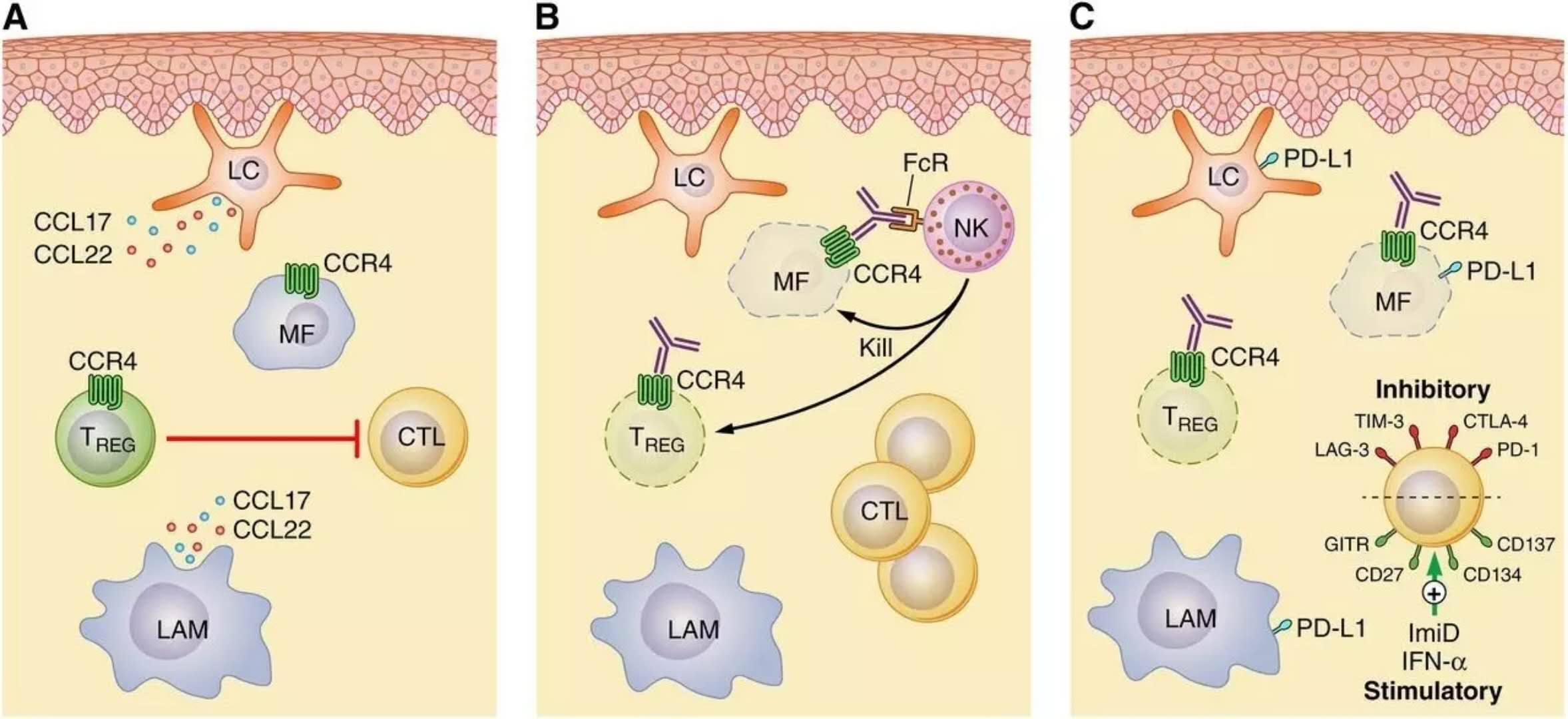
â–²CCR4 is a therapeutic target for cutaneous T-cell lymphoma (Source: Blood Journal)
The efficacy of Mogamulizumab was validated in a phase 3 clinical trial MAVORIC. This open-label, multicenter, randomized trial evaluated the efficacy of mogamulizumab in patients with MF and SS who had failed after at least one systemic treatment. It recruited 372 patients in the United States, Europe, Japan, and Australia. The top line results demonstrated a significant improvement in progression-free survival (PFS) in patients treated with mogamulizumab compared to the control group, while being well tolerated.
“I am very pleased that the FDA has accepted the BLA of mogamulizumab and granted priority review, which is another major achievement of our subsidiary Kyowa Kirin Pharmaceutical Development,†said Dr. Mitsuo Satoh, Vice President and Executive Director of Kyowa Hakko Kirin. We will continue to work with regulatory agencies such as the FDA to provide this drug to CTCL patients in the United States as soon as possible."
Reference materials:
[1] Kyowa Hakko Kirin Announces FDA Acceptance for Filing and Priority Review Designation of Mogamulizumab's Biologics License Application
[2] Kyowa Hakko Kirin Official Website
Water System,Water Supply System,Feed Water System,Boiler Feed System
JIANGSU NEW FIRE FIGHTING TECHNOLOGY CO.,LTD , https://www.newayfire.com